The United States Pharmacopeia (USP) released an updated version of USP 797, the standard for pharmaceutical compounding of sterile preparations in November 2023.(1) Compliance with this standard is crucial to promote patient safety, prevent adverse events and avoid medication preparation or administration mistakes. Further, USP 797 is enforceable by state and federal regulatory agencies(2, 3) and is included as part of The Joint Commission accreditation surveys.(4) Non-compliance with sterile compounding guidelines has been associated with patient morbidities and mortalities, fines, and facility shutdowns.(5-11) Therefore, it is imperative that sterile compounding facilities are designed properly and personnel receive appropriate training and equipment to comply with USP 797 requirements.
What is a PEC?
Consistently, USP 797 requirements have emphasized the importance of the sterile compounding environment and the use of proper equipment. According to USP 797 all sterile compounding activities must be completed using a PEC or primary engineering control. A PEC is a device, area, or enclosure that provides ISO Class 5 or better air quality using a unidirectional airflow and high efficiency particulate air (HEPA)-filtered air. Generally, PECs can be split into three categories:
-
Laminar airflow systems use a horizontal or vertical laminar airflow to provide an adequate compounding environment. Laminar airflow systems can include laminar airflow workbenches, integrated vertical laminar flow zones, and Class II biological safety cabinets.
-
Restricted-access barrier system including compounding aseptic isolators and compounding aseptic containment isolators are enclosures that use defined openings for the input and removal of materials into the enclosure. These openings are then closed for the duration of the compounding process and the components are manipulated through glove ports.
-
Pharmaceutical isolators are similar to restricted-access barrier systems, but are completely sealed off from the outside environment and often contain an integrated decontamination system.
Regardless of the type of PEC, integrated HEPA filters remove microbial contaminants and dust particles from the air before it enters the workspace. The unidirectional airflow then introduces this clean filtered air to the work space, while removing contaminated air. Together, HEPA filtration and unidirectional airflow reduce the risk for microbial or dust/particle contamination as well as chemical cross contamination in the final compounded product.
Sterile compounding PECs require consistent maintenance and monitoring
USP 797 requires all PECs used for sterile compounding be properly certified and maintained. Once the PEC is setup within the cleanroom or segregated compounding area (SCA), it must be certified prior to use. Certification requires testing for the airflow, HEPA filter integrity, and total particle count to ensure the unit is providing adequate air quality, velocity, and flow. A dynamic airflow smoke pattern test must also be completed to demonstrate a unidirectional airflow is achieved within the PEC. Once initially certified, the PEC must be recertified at least every six months as well as every time the cleanroom or SCA setup or placement of equipment changes.
Following certification, PECs must be monitored and maintained to ensure proper functionality and reduce the likelihood of contamination. The frequency of maintenance and monitoring activities is determined by the category of CSP that is being compounded. CSPs are divided into three categories (1, 2, and 3), where each category is defined by storage times and conditions of the final compounded product. Category 1 presents the least risk for adverse events, while category 3 CSPs present the most risk. The PEC should be cleaned and disinfected every day that compounding takes place and whenever there is known or suspected contamination of the PEC surface. Further, a sporicidal disinfectant should be applied at least monthly for category 1 and 2 CSPs or weekly for category 3 CSPs. All cleaners and disinfectants must remain in contact with the PEC surface for the time specified in the manufacture's instructions for use. To demonstrate that cleaning and disinfection procedures are adequate and PEC airflow and use conditions are providing a low risk environment, the surfaces and air within a PEC should be sampled for viable particle counts at least every six months for category 1 and 2 CSPs. Facilities that compound category 3 CSPs must complete air and surface sampling within 30 days prior to starting compounding and then at least monthly. Table 1 provides an overview of certification, maintenance and monitoring activities required for each CSP category.
Table 1. Summary of PEC certification, maintenance, and monitoring requirements.*
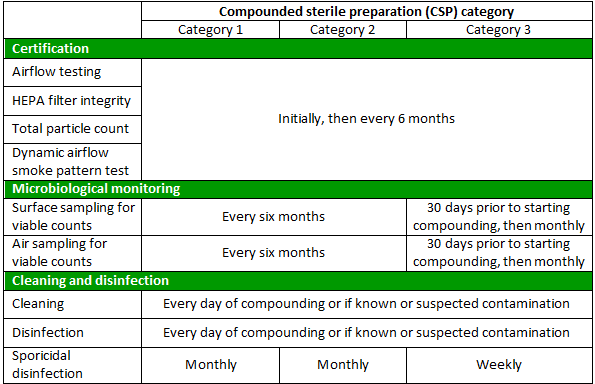
*Table provides maximum testing intervals allowed.
More frequent testing or maintenance may be desired or required by facility standard operating procedures.
Mystaire can help you establish and maintain PEC compliance
The requirements for PEC functionality and maintenance to comply with USP 797 are numerous. However, Mystaire ductless enclosures can make USP 797 PEC compliance a little easier. Mystaire ductless enclosures including vertical and horizontal laminar flow hoods meet USP 797 airflow and air quality requirements. These enclosures can be used as PECs for compounding any category of non-hazardous/non-antineoplastic medications. The unidirectional laminar airflow and built-in HEPA filtration provide air quality that meets ISO 5 specifications for sterile compounding. Built-in sensors sound an audible alarm when airflow within the unit does not reach preprogrammed velocities to prevent personnel from working with subpar air flow, which could lead to contamination of sterile preparations. Further, the ductless nature provides placement flexibility within a cleanroom or SCA to minimize disruptions to airflow and pressure differentials within the compounding area. All enclosures are composed of a smooth non-sorptive and non-reactive polypropylene and/or polycarbonate shell and work surface to promote cleanability. These materials are compatible with a variety of cleaners and disinfectants including Medi-Fect™ disinfectant wipes and Solucide® hard surface disinfectant spray. Widespread cleaner and disinfectant compatibility provides flexibility to choose the cleaner(s) and disinfectant(s) that work best for each facility. The thoughtful design and built in safeguards within Mystaire ductless enclosures can provide a straightforward solution to help facilities comply with the PEC requirements specified in USP 797.
References
-
United States Pharmacopeia. General Chapter <797> Pharmaceutical Compounding – Sterile Preparations. 2023.
-
Food and Drug Administration. “Pharmacy Compounding of Human Drug Products Under Section 503A of the Federal Food, Drug, and Cosmetic Act.” Rev. 2. 2016.
-
United States Pharmacopeia. USP Quality Standards for Compounding. Accessed January 4, 2024.
https://www.usp.org/sites/default/files/usp/document/about/usp-quality-standards-for-compounding.pdf -
The Joint Commission. “Prepublication Requirements: Revised Medication Compounding Requirements.” 2023.
https://www.jointcommission.org/-/media/tjc/documents/standards/prepublications/effective-2023/mdc_edits_prepub_jan2024.pdf -
Centers for Disease Control and Prevention. Multistate Meningitis Outbreak. 2015. Accessed January 2, 2024.
https://www.cdc.gov/hai/outbreaks/meningitis.html -
Food and Drug Administration. Federal Judge Enters Consent Decree Against Texas Compounder, Guardian Pharmacy Services. 2019. Accessed January 2, 2024.
https://www.fda.gov/drugs/human-drug-compounding/fdas-investigation-guardians-compounded-triamcinolone-moxifloxacin-drug-product -
Food and Drug Administration. FDA’s Investigation into Guardian’s Compounded triamcinolone-moxifloxacin drug product. 2018. Accessed January 2, 2024. Accessed January 2, 2024.
https://www.fda.gov/news-events/press-announcements/federal-judge-enters-consent-decree-against-texas-compounder-guardian-pharmacy-services -
Food and Drug Administration. Federal judge enters consent decree against Specialty Compounding LLC. 2015. Accessed January 4, 2024.
https://web.archive.org/web/20170112025127/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm437682.htm -
How Sterile Compounding has Evolved and Actions Taken. 2015. Accessed January 2, 2024.
https://www.wolterskluwer.com/en/expert-insights/a-timeline-of-sterile-compounding-events-and-actions-taken -
Shehab N, Brown MN, Kallen AJ, Perz JF. U.S. Compounding Pharmacy-Related Outbreaks, 2001-2013: Public Health and Patient Safety Lessons Learned. J Patient Saf. 2018 Sep;14(3):164-173.
-
U.S. Senate Committee on Health Education, Labor, & Pensions. FDA Alert – Sterile Products from Specialty Compounding in Cedar Park, TX. 2013.
https://www.help.senate.gov/ranking/newsroom/press/fda-alert-sterile-products-from-specialty-compounding-in-cedar-park-tx